Attentive gardeners notice that sometimes vegetable and fruit crops are in no hurry to please with a rich harvest and constantly suffer from some diseases and pests. And all this-despite the care and regularly applied top dressing. What is the reason? One of the factors that have a fundamental influence on the growth and development of plants is the acidity of the soil.
Whoever the gardener is by profession and education in the main life, on his acres he involuntarily becomes an agronomist and a specialist in the field of plant physiology, a chemist and a phytopathologist. But what about the other way? The quantity and quality of the fruit, the decorative nature of the flower beds and the long life of the trees depend on the conditions created by the owner of the garden. Let’s look at what acidity is, how it affects plants, and what are the preferences of different crops regarding this soil characteristic.
Pundus hydrogenium: acidic, alkaline or neutral?
Probably, everyone has heard the letter combination pH. Most often, they talk about it in commercials for cosmetic products. The hydrogen pH index (an abbreviation of the Latin pondus Hydrogenii — “weight of hydrogen”) is an important characteristic of the acid-base properties of various aqueous solutions and biological fluids. Many chemical processes in nature and living organisms depend on the level of acidity of the environment.
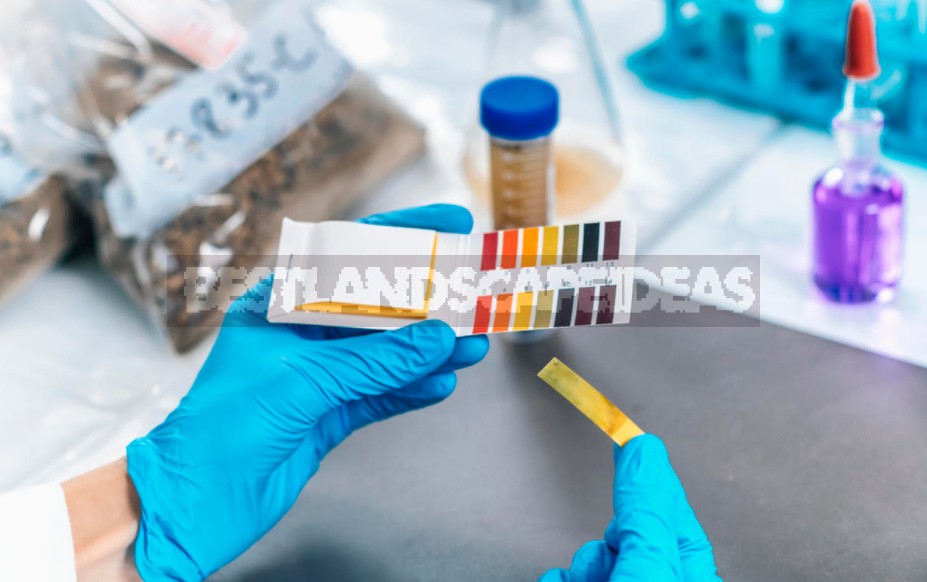
Pundus hydrogenium is a quantitative measure of the ratio of H⁺ and OH-ions formed during the dissociation (decomposition) of water molecules. If the amount of H⁺ and OH-is equal (as it happens in perfectly pure distilled water), then we speak of a neutral reaction. If free hydrogen ions (H⁺) predominate, then the medium is acidic. And vice versa: if there are more hydroxide ions (OH -) in the solution, then it is an alkali.

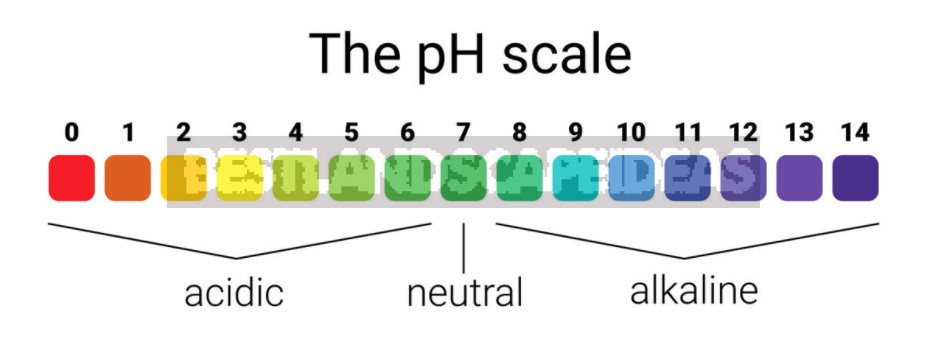
The relative amount of hydrogen ions H⁺ expresses the degree of acidity or alkalinity of the solution in pH units. Acidity and alkalinity are measured on a special scale with values from 0 to 14, where 7 corresponds to neutral solutions. What has an acidity index below 7 is acidic, and what is higher is alkaline. The acidity scale is logarithmic. This means that the acidity, increasing by one, changes tenfold. For example, the concentration of H⁺ in a solution with pH = 5 is 10 times greater than that of a solution with pH = 6, and 100 times greater than in distilled water with pH = 7.
The hydrogen index is of great importance for living organisms — pH affects the course of all biochemical processes. So, for example, we feel the pain of a cut or burn “thanks” to the hydrogen cations H⁺. They change the acidity of the neutral solution of the intercellular space, getting there from the intracellular fluid of damaged cells. Changes in acidity and “feel” the nerve endings.
Effect of soil solution acidity on plants
Like other living organisms, plants also depend on the acid-base balance. Their feeding process, in addition to photosynthesis, is based on ion-cation exchange between plant cells and soil solution. The absorption of nutrient ions from the soil by the green organism depends on the reaction of the medium (acidic or alkaline). The most powerful effect of the acidic environment has on plants at their young age.

The influence of the acidic or alkaline environment of the soil on the green inhabitants is diverse.
- In acidic soil, the growth of the root system deteriorates. As a result, plants do not receive enough nutrients.
- In acidic and alkaline environments, the absorption of some elements increases and the absorption of others decreases. This means that the plant has a deficiency of certain elements. As can be seen from the diagram below, the optimal conditions for plant nutrition are in an environment that has a reaction close to neutral.
- The high acidity of the soil also acidifies the cellular juice of plants, which affects the internal biochemical processes. Protein synthesis decreases — the quality of fruits deteriorates, and the process of converting monosaccharides into sucrose is also suppressed.
- Exposure to an acidic environment at an early stage of development significantly disrupts the carbohydrate and protein metabolism of the plant, which affects the laying of generative organs and the subsequent formation of fruits.
- In soils with a low pH value, many substances (for example, Fe, Mn, Zn, Cu) become toxic to plants.

- In acidic soils, not only plants, but also microorganisms feel bad. There are almost no earthworms, the processes of nitrogen fixation and nitrification are sluggish, which again negatively affects the nutrition of plants.
- In acidic soils, hydrogen displaces calcium, which leads to the grinding of soil particles. Such soils have a poor structure, low moisture capacity.
Who doesn’t feel sour?
Different plants react differently to the pH of the soil. That is why our planet is green, despite the increased acidity or alkalinity in some regions: wild species adapt to any conditions. Botanists have compiled special lists that classify plants according to their needs for moisture, nitrogen, light, and soil acidity. However, most garden and vegetable crops prefer a slightly acidic or neutral (pH 6-7) soil reaction.

By the way, in most of the country, the soil is acidic: in the temperate-cold zone, precipitation prevails over evaporation and transpiration (the movement of water through the plant). Therefore, the salts of calcium, magnesium, potassium and sodium, which are necessary to neutralize the acidic decomposition products of leaf litter, are washed out of the root layer into the underlying layers.
Soils are divided according to the level of acidity pH:
- for strongly acidic-less than 4.5,
- acidic-4.6-5,
- slightly acidic-5.1-5.5,
- close to neutral-5.6-6,
- neutral-6.1-7,
- slightly alkaline-7.1-8.
Garden plants can be divided into 3 groups, having a different pH range, in which they develop most well:
- acidophils (from 5 to 6 pH),
- neutrophils (from 6 to 7 pH),
- basiphils (from 7 to 7.5 pH).
You can see the table of optimal acidity for the most common vegetable and fruit crops. Some plants can normally exist in different categories, while changing their properties depending on the acidity of the environment. The most striking example is Hydrangea macrophylla. It changes the color of its flowers from blue in acidic soil (pH = 4.5-5) to white-beige in neutral (pH = 6-7) and pink in alkaline (pH = 6.5-8).
Obviously, knowing the reaction of your soil is very important for successful gardening and gardening. However, a certain pH is not a verdict.


















